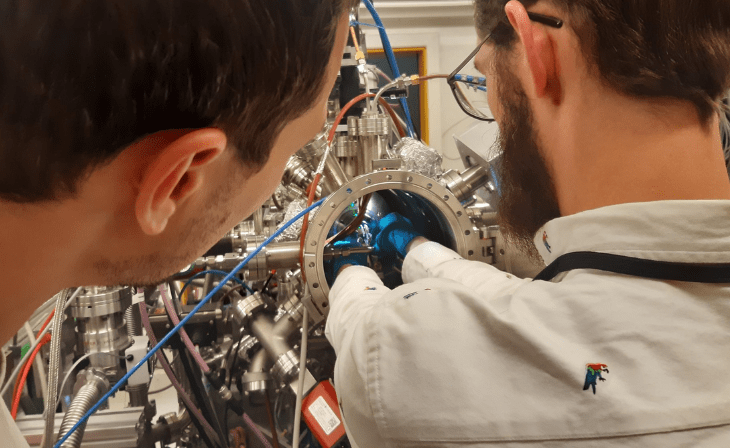
Raffael Rameshan from the Chair of Physical Chemistry at the University of Leoben and an international research team have been able to demonstrate an improvement in ammonia production. These findings were recently published in the renowned scientific journal “Nature”. They can help to significantly reduce the high carbon dioxide emissions in the production of this important chemical compound. Ammonia is required in the production of fertilisers and is a promising alternative to the fossil fuels used to date.
The Haber-Bosch process is a large-scale industrial chemical process for synthesising ammonia. It is named after the German chemists Fritz Haber and Carl Bosch, who developed the process at the beginning of the 20th century. The central step of the process, the synthesis of ammonia from atmospheric nitrogen and hydrogen, is carried out on an iron-containing catalyst at pressures of around 150 to 350 bar and temperatures of around 400 to 500 degrees Celsius.
Most of the ammonia (around 80 per cent) is required for fertiliser production, which means it also ensures that the world’s population is fed. In addition, around 3 per cent of the energy produced worldwide is needed to manufacture ammonia (which means it makes a significant contribution to global CO2 emissions). Even small improvements to the Haber-Bosch process, in particular the catalyst used, therefore have a major impact on CO2 emissions and energy consumption.
“Although the process has been in use for over 100 years, the catalyst is still being feverishly researched and the exact mechanism has not yet been fully unravelled. Scientists have been cutting their teeth on this for decades. The crux of the matter is to make the nitrogen reactive so that it can then be transformed into ammonia with hydrogen,” says Raffael Rameshan from the University of Leoben, explaining the challenge.